Aflibercept
Description
This is a recombinant protein using the same sequences as the therapeutic protein aflibercept. It consists of the binding domains from two human vascular endothelial growth factor (VEGF) receptors, VEGFR1 and VEGFR2, fused with the Fc region of human immunoglobulin gamma 1 (IgG1). By incorporating both VEGFRs, aflibercept exhibits enhanced affinity to the cognate ligands compared to the endogenous individual receptor. However, it lacks the intracellular structure necessary for subsequent signal transduction, thus effectively sequestering the ligands and preventing activation of VEGFR. Ziv-aflibercept, marketed as Zaltrap, was developed as an intravenous injection for the treatment of metastatic colorectal cancer. The intravitreal formulation known as Eyelea, has been approved for the treatment of wet age-related macular degeneration, diabetic macular edema, and diabetic retinopathy.
Product name | Aflibercept Biosimilar |
Species | Homo sapiens |
Expression system | CHO-K1 |
Buffer | PBS, pH 7.4 |
Delivery condition | Dry ice (-80°C) |
Delivery Time | 1 week if in stock; 4 weeks if production needed |
Storage condition | Store at -80°C |
Brand | BioMetas |
Applications | ELISA, assay, in vivo |
Aliases/Synonyms | Zaltrap, Eylea, VEGF Trap, VEGF Trap R1/R2, Ziv-aflibercept, AVE005, AVE0005, Bay865321 |
Reference | |
Note | For research use only. Not suitable for clinical or therapeutic use. |
Type | VEGFR Fc (hIgG1) fusion |
Clonality |
Size | 1mg, 5mg, 10mg, 50mg, 100mg |
Brand | BioMetas |
Product type | Biosimilar |
Clonality | Monoclonal Antibody |
Expression system | CHO stable pool |
Applications | Elisa, assay, in vivo |
| Amount | Price |
| 1mg | ¥1200 |
| 5mg | ¥3000 |
| 10mg | ¥5000 |
| 25mg | ¥7500 |
| 50mg | ¥10000 |
| 100mg | ¥15000 |
Contact Us for a Quote!
Data Gallery
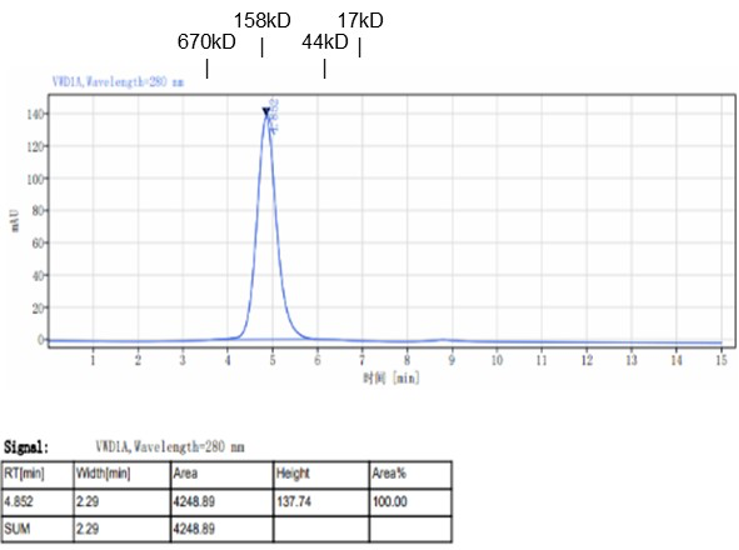
Fig. 1.) 4-20% SDS-PAGE analysis
Recombinant protein was visualized by Coomassie Brilliant Blue R250 staining.
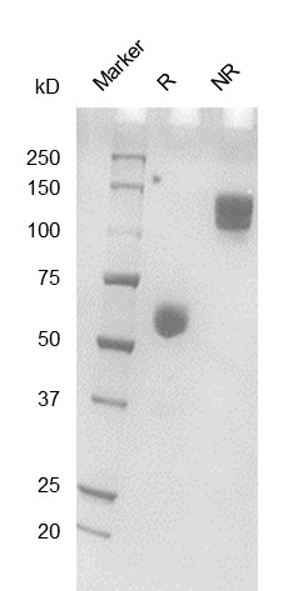
Fig. 2.) SEC-HPLC analysis
Column: Superdex 200 Increase 5/150 GL
Running buffer: 2xPBS, pH 7.4