Eculizumab
Description
This is a humanized IgG4 antibody using the same sequences as the therapeutic antibody eculizumab. It targets complement protein C5, inhibiting its cleavage into C5a and C5b, and the formation of the terminal complement complex C5b-9. By blocking this complex, eculizumab successfully prevents complement-mediated intravascular hemolysis in paroxysmal nocturnal hemoglubunuria (PNH), complement-mediated microangiopathy in atypical hemolytic uremic syndrome (aHUS), as well as immune-mediated inflammation and damage of the central nervous system in neuromyelitis optica spectrum disorder. Eculizumab has a long duration of action. It was the first drug approved for each of its indications, and its approval was granted based on small-scale clinical trials.
Product name | Eculizumab Biosimilar |
Species | Homo sapiens |
Expression system | CHO-K1 |
Buffer | PBS, pH 7.4 |
Delivery condition | Dry ice (-80°C) |
Delivery Time | 1 week if in stock; 4 weeks if production needed |
Storage condition | Store at -80°C |
Brand | BioMetas |
Applications | ELISA, assay, in vivo |
Aliases/Synonyms | Soliris, 5G1.1, h5G1.1, h5G1.1HuG2/G4, H5G1-1, H5G11 |
Reference | |
Note | For research use only. Not suitable for clinical or therapeutic use. |
Isotype | IgG4 |
Clonality | Monoclonal Antibody |
Size | 1mg, 5mg, 10mg, 50mg, 100mg |
Brand | BioMetas |
Product type | Biosimilar |
Clonality | Monoclonal Antibody |
Expression system | CHO-K1 |
Applications | Elisa, assay, in vivo |
| Amount | Price |
| 1mg | ¥1200 |
| 5mg | ¥3000 |
| 10mg | ¥5000 |
| 25mg | ¥7500 |
| 50mg | ¥10000 |
| 100mg | ¥15000 |
Contact Us for a Quote!
Data Gallery
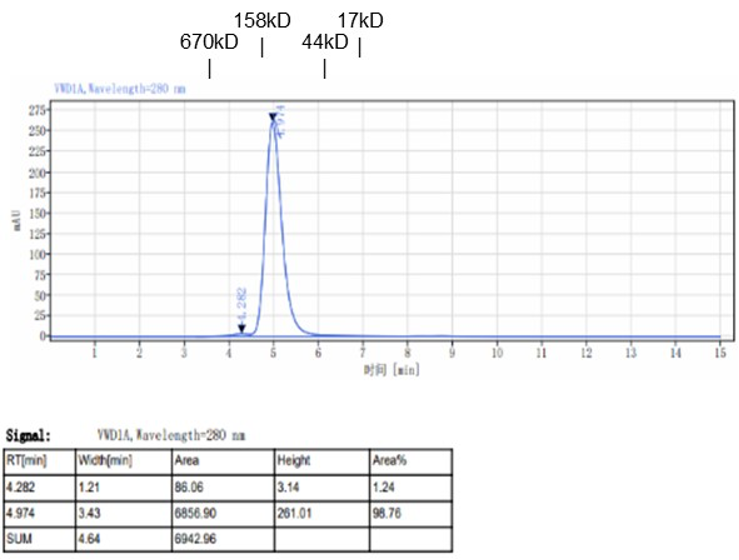
Fig. 1.) 4-20% SDS-PAGE analysis
Recombinant protein was visualized by Coomassie Brilliant Blue R250 staining.
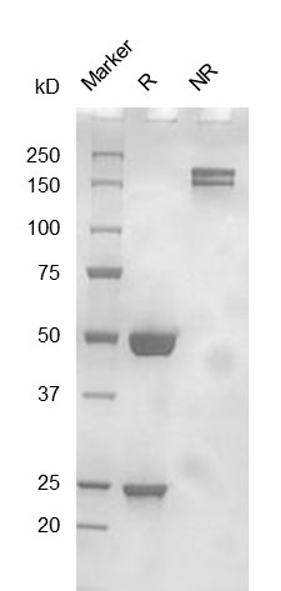
Fig. 2.) SEC-HPLC analysis
Column: Superdex 200 Increase 5/150 GL
Running buffer: 2xPBS, pH 7.4