Inebilizumab
Description
This is a humanized IgG1 antibody using the same sequences as the therapeutic antibody inebilizumab. It is cytolytic, directed against the broadly epressed B-cell surface antigen CD19, resulting in B-cell depletion in autoimmune conditions. Inebilizumab is indicated for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adult patients who are anti-aquaporin-4 (AQP4) antibody positive. NMOSD is a rare autoimmune disorder in which immune system cells and autoantibodies attack and damage the optic nerves and spinal cord. In most people with NMOSD, B cells produce antibodies that attack AQP4, a protein involved in nverve cell function, causing inflammation and damage to the central nervous system. By reducing the numbers of B cells, the medicine is expected to prevent damage to nerve cells and reduce the symptoms of the condition.
Product name | Inebilizumab Biosimilar |
Species | Homo sapiens |
Expression system | CHO-K1 |
Buffer | PBS, pH 7.4 |
Delivery condition | Dry ice (-80°C) |
Delivery Time | 1 week if in stock; 4 weeks if production needed |
Storage condition | Store at -80°C |
Brand | BioMetas |
Applications | ELISA, assay, in vivo |
Aliases/Synonyms | Uplizna, MEDI-551, inebilizumab-cdon |
Reference | |
Note | For research use only. Not suitable for clinical or therapeutic use. |
Isotype | IgG1 |
Clonality | Monoclonal Antibody |
Size | 1mg, 5mg, 10mg, 50mg, 100mg |
Brand | BioMetas |
Product type | Biosimilar |
Clonality | Monoclonal Antibody |
Expression system | CHO-K1 |
Applications | Elisa, assay, in vivo |
| Amount | Price |
| 1mg | ¥1200 |
| 5mg | ¥3000 |
| 10mg | ¥5000 |
| 25mg | ¥7500 |
| 50mg | ¥10000 |
| 100mg | ¥14000 |
Contact Us for a Quote!
Data Gallery
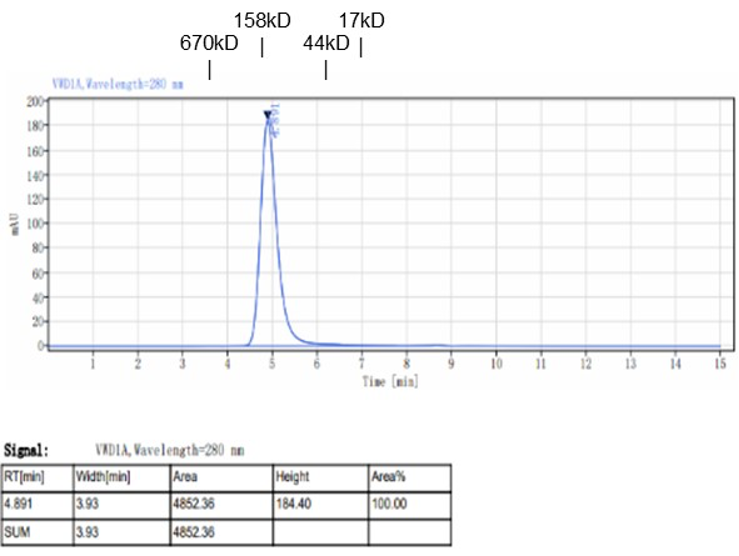
Fig. 1.) 4-20% SDS-PAGE analysis
Recombinant protein was visualized by Coomassie Brilliant Blue R250 staining.
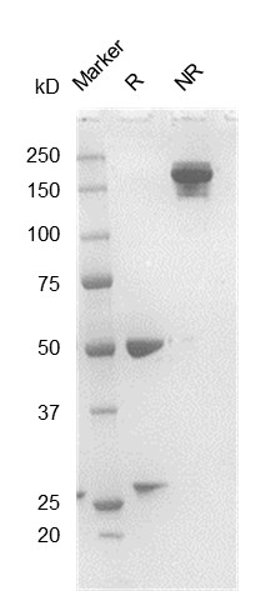
Fig. 2.) SEC-HPLC analysis
Column: Superdex 200 Increase 5/150 GL
Running buffer: 2xPBS, pH 7.4