Golimumab
Description
This is a fully human IgG1 monoclonal antibody using the same sequences as the therapeutic antibody golimumab. It is derived from immunizing genetically engineered mice with human tumor necrosis factor alpha (TNFa). Elevated TNFa levels are associated with chronic inflammation. Golimumab binds and inhibits both soluble and transmembrane hTNFa, thereby preventing its interaction with receptors. This inhibition impedes leukocyte infiltration by blocking cell adhesion proteins such as E-selectin, ICAM-1 and VCAM-1, as well as suppressing pro-inflammatory cytokine secretion including IL6, IL8, G-CSF and GM-CSF in vitro. Therefore, golimumab is indicated for use in adults as an adjunct to methotrexate treatment in patients with moderate to severe active rheumatoid arthritis (RA), alone or as an adjunct to methotrexate treatment in patients with active psoriatic arthritis (PsA), as a single agent in patients with active ankylosing spondylitis (AS), and also as a single agent in patients with moderate to severe ulcerative colitis (UC) who require chronic steroids or have experienced intolerance or only a partial response to previous medications.
Product name | Golimumab Biosimilar |
Species | Homo sapiens |
Expression system | CHO-K1 |
Buffer | PBS, pH 7.4 |
Delivery condition | Dry ice (-80°C) |
Delivery Time | 1 week if in stock; 4 weeks if production needed |
Storage condition | Store at -80°C |
Brand | BioMetas |
Applications | ELISA, assay, in vivo |
Aliases/Synonyms | Simponi®, Simponi Aria®, CNTO148 |
Reference | |
Note | For research use only. Not suitable for clinical or therapeutic use. |
Isotype | IgG1 |
Clonality | Monoclonal Antibody |
Size | 1mg, 5mg, 10mg, 50mg, 100mg |
Brand | BioMetas |
Product type | Biosimilar |
Clonality | Monoclonal Antibody |
Expression system | CHO-K1 |
Applications | Elisa, assay, in vivo |
| Amount | Price |
| 1mg | ¥1200 |
| 5mg | ¥3000 |
| 10mg | ¥5000 |
| 25mg | ¥7500 |
| 50mg | ¥10000 |
| 100mg | ¥15000 |
Contact Us for a Quote!
Data Gallery
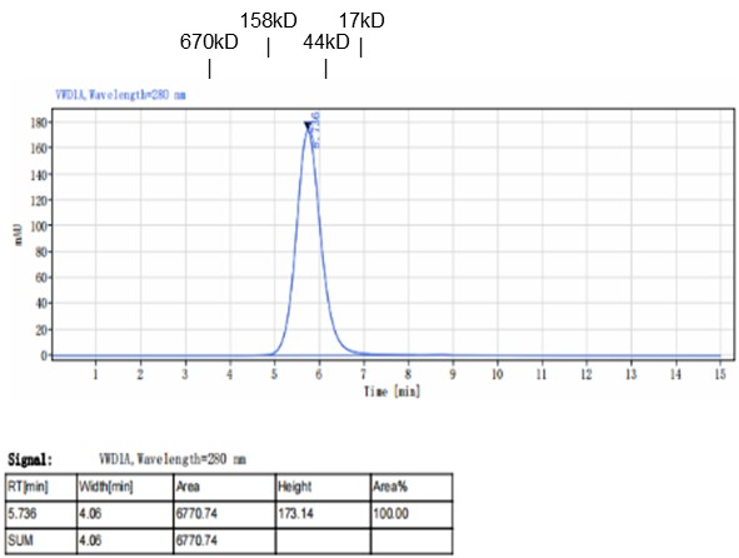
Fig. 1.) 4-20% SDS-PAGE analysis
Recombinant protein was visualized by Coomassie Brilliant Blue R250 staining.
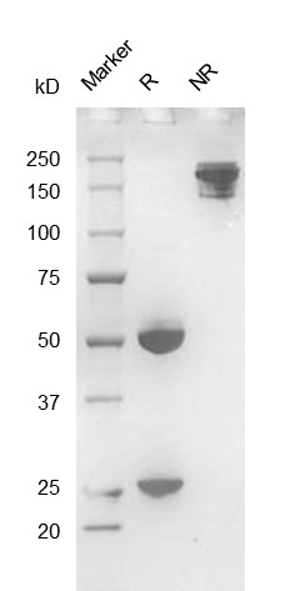
Fig. 2.) SEC-HPLC analysis
Column: Superdex 200 Increase 5/150 GL
Running buffer: 2xPBS, pH 7.4