Ivonescimab
Description
Ivonescimab is a first-in-class PD-1/VEGF bispecific antibody under investigation in phase 3 clinical trial. Engineered with Tetrabody technology, ivonescimab blocks PD-1 binding to PD-L1 and PD-L2, and blocks VEGF binding to VEGF receptors. PD-1 inhibitors combined with VEGF inhibitors have shown robust efficacy in several tumor types, including renal cell carcinoma, NSCLC, and hepatocellular carcinoma. The use of ivonescimab monotherapy to inhibit PD-1 and VEGF co-expression in the tumor microenvironment may block these 2 pathways more effectively and enhance the antitumor activity compared with combination therapy.
Product name | Ivonescimab Biosimilar |
Species | Homo sapiens |
Expression system | CHO-K1 |
Buffer | PBS, pH 7.4 |
Delivery condition | Dry ice (-80°C) |
Delivery Time | 1 week if in stock; 4 weeks if production needed |
Storage condition | Store at -80°C |
Brand | BioMetas |
Applications | ELISA, assay, in vivo |
Aliases/Synonyms | AK104, AK-104 |
Reference | |
Note | For research use only. Not suitable for clinical or therapeutic use. |
Isotype | IgG1, κ |
Clonality | Monoclonal Antibody |
Size | 1mg, 5mg, 10mg, 50mg, 100mg |
Brand | BioMetas |
Product type | Biosimilar |
Clonality | Monoclonal Antibody |
Expression system | CHOK1 |
Applications | Elisa, assay, in vivo |
Contact Us for a Quote!
Data Gallery
Fig. 1.) 4-20% SDS-PAGE analysis
Recombinant protein was visualized by Coomassie Brilliant Blue R250 staining.
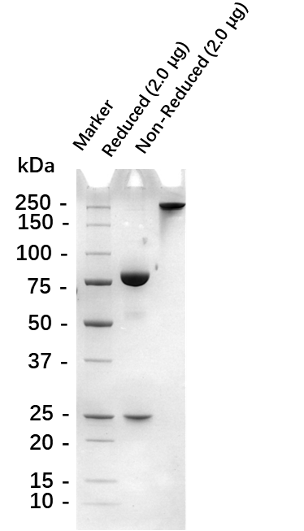
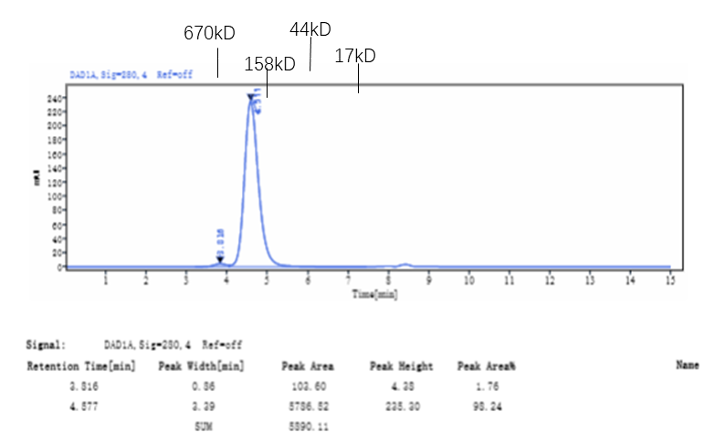
Fig. 2.) SEC-HPLC analysis
Column: Superdex 200 Increase 5/150 GL
Running buffer: 2xPBS, pH 7.4