Ixekizumab
Description
This is a humanized IgG4 antibody using the same sequences as the therapeutic antibody ixekizumab. It specifically targets interleukin-17A (IL17A) and prevents its interaction with the IL17A receptor. Given that IL17A is a pro-inflammatory cytokine involved in inflammation and immune responses, blocking its activity holds significant therapeutic potential for inflammatory conditions. Notably, IL17A stimulates proliferation and activation of keratinocytes in the skin. Ixekizumab is indicated for treating patients aged six years or older with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Additionally, it is also indicated in adult patients with active psoriatic arthritis, ankylosing spondylitis, or non-radiographic axial spondyloarthritis accompanied by objective signs of inflammation.
Product name | Ixekizumab Biosimilar |
Species | Homo sapiens |
Expression system | CHO-K1 |
Buffer | PBS, pH 7.4 |
Delivery condition | Dry ice (-80°C) |
Delivery Time | 1 week if in stock; 4 weeks if production needed |
Storage condition | Store at -80°C |
Brand | BioMetas |
Applications | ELISA, assay, in vivo |
Aliases/Synonyms | Taltz®, LY2439821 |
Reference | |
Note | For research use only. Not suitable for clinical or therapeutic use. |
Isotype | IgG4 |
Clonality | Monoclonal Antibody |
Size | 1mg, 5mg, 10mg, 50mg, 100mg |
Brand | BioMetas |
Product type | Biosimilar |
Clonality | Monoclonal Antibody |
Expression system | CHO-K1 |
Applications | Elisa, assay, in vivo |
| Amount | Price |
| 1mg | ¥2400 |
| 5mg | ¥6000 |
| 10mg | ¥8000 |
| 25mg | ¥12000 |
| 50mg | ¥15000 |
| 100mg | ¥25000 |
Contact Us for a Quote!
Data Gallery
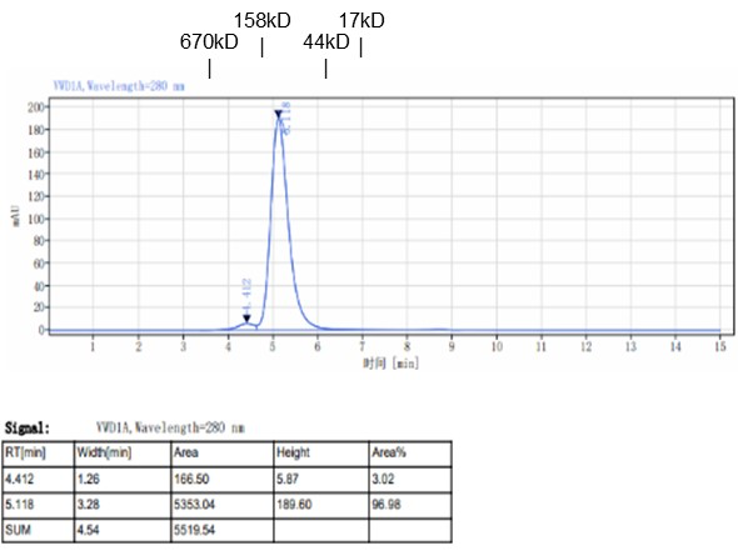
Fig. 1.) 4-20% SDS-PAGE analysis
Recombinant protein was visualized by Coomassie Brilliant Blue R250 staining.
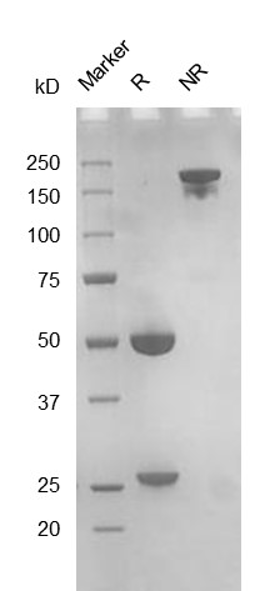
Fig. 2.) SEC-HPLC analysis
Column: Superdex 200 Increase 5/150 GL
Running buffer: 2xPBS, pH 7.4