Imvotamab
Description
This is an IgM-based CD20xCD3 bispecific antibody T cell engager (TCE) for the treatment of B cell non-Hodgkin’s lymphoma (NHL). Preclinical research demonstrates that imvotamab may have advantages over IgG bispecific antibodies including greater binding power to CD20 expressing cancer cells especially when CD20 expression has been reduced due to prior treatment with anti-CD20 antibodies. It has also been shown to have good target cell killing efficacy combined with a lower cytokine release profile associated with the T cell directed cellular cytotoxicity (TDCC) mechanism of action compared to an IgG based CD20 x CD3 antibody with the same CD20 and CD3 binding units, when tested in vitro with human T cells and human B cells.
Product name | Imvotamab Biosimilar |
Species | Homo sapiens |
Expression system | CHO-K1 |
Buffer | PBS, pH 7.4 |
Delivery condition | Dry ice (-80°C) |
Delivery Time | 1 week if in stock; 4 weeks if production needed |
Storage condition | Store at -80°C |
Brand | BioMetas |
Applications | ELISA, assay, in vivo |
Aliases/Synonyms | IGM-2323, IGM 2323, IGM2323 |
Reference | |
Note | For research use only. Not suitable for clinical or therapeutic use. |
Isotype | IgM |
Clonality | Monoclonal Antibody |
Size | 1mg, 5mg, 10mg, 50mg, 100mg |
Brand | BioMetas |
Product type | Biosimilar |
Clonality | Monoclonal Antibody |
Expression system | CHO-K1 |
Applications | Elisa, assay, in vivo |
| Amount | Price |
| 1mg | ¥4000 |
| 5mg | ¥10000 |
| 10mg | ¥15000 |
| 25mg | ¥30000 |
| 50mg | ¥50000 |
| 100mg | ¥100000 |
Contact Us for a Quote!
Data Gallery
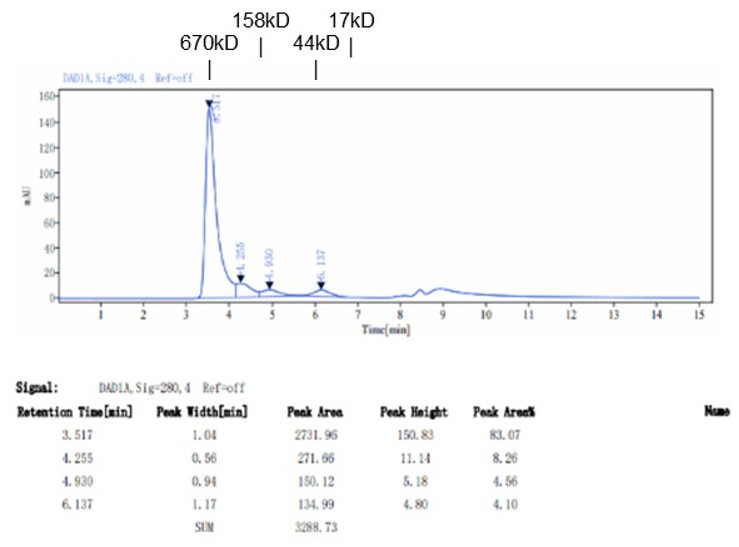
Fig. 1.) 4-20% SDS-PAGE analysis
Recombinant protein was visualized by Coomassie Brilliant Blue R250 staining.
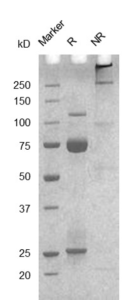
Fig. 2.) SEC-HPLC analysis
Column: Superdex 200 Increase 5/150 GL
Running buffer: 2xPBS, pH 7.4