Ravulizumab
Description
This is a humanized IgG2/4 monoclonal antibody using the same sequences as the therapeutic antibody ravulizumab. It exhibits potent and selective inhibition of complement 5 (C5). Ravulizumab was engineered from eculizumab, another complement inhibitor, with the aim of prolonging the duration of action and reducing dosing frequency. It inhibits the terminal complement pathway by binding to C5 with high affinity, thereby inhibiting the cleavage of C5 into pro-inflammatory and pro-thrombotic anaphylatoxin C5a, as well as C5b, an initiating subunit of the terminal complement complex C5b-9, which promotes cell lysis. By blocking the generation of C5b, the formation of C5b-9 is also prevented. In patients with paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS), ravulizumab effectively inhibits terminal complement-mediated intravascular hemolysis and complement-mediated thrombotic microangiopathy (TMA) respectively. Additionally, through blocking the complement system, it mitigates the extent of inflammatory and immune responses that contribute to the pathophysiology of myasthenia gravis.
Product name | Ravulizumab Biosimilar |
Species | Homo sapiens |
Expression system | CHO-K1 |
Buffer | PBS, pH 7.4 |
Delivery condition | Dry ice (-80°C) |
Delivery Time | 1 week if in stock; 4 weeks if production needed |
Storage condition | Store at -80°C |
Brand | BioMetas |
Applications | ELISA, assay, in vivo |
Aliases/Synonyms | Ultomiris™, ALXN-1210, ravulizumab-cwvz |
Reference | |
Note | For research use only. Not suitable for clinical or therapeutic use. |
Isotype | IgG2/4 |
Clonality | Monoclonal Antibody |
Size | 1mg, 5mg, 10mg, 50mg, 100mg |
Brand | BioMetas |
Product type | Biosimilar |
Clonality | Monoclonal Antibody |
Expression system | CHO-K1 |
Applications | Elisa, assay, in vivo |
| Amount | Price |
| 1mg | ¥1200 |
| 5mg | ¥3000 |
| 10mg | ¥5000 |
| 25mg | ¥7500 |
| 50mg | ¥10000 |
| 100mg | ¥14000 |
Contact Us for a Quote!
Data Gallery
Fig. 1.) 4-20% SDS-PAGE analysis
Recombinant protein was visualized by Coomassie Brilliant Blue R250 staining.
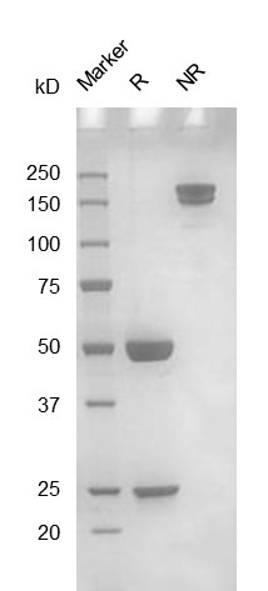
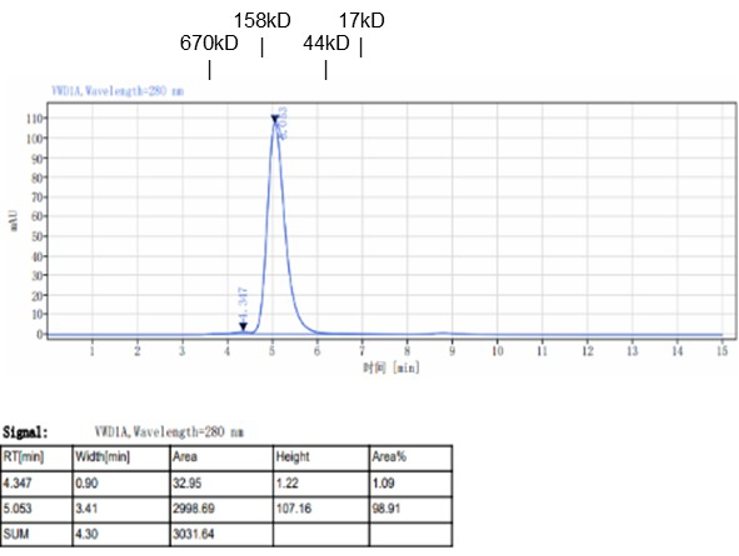
Fig. 2.) SEC-HPLC analysis
Column: Superdex 200 Increase 5/150 GL
Running buffer: 2xPBS, pH 7.4